Hydrogen: The lifeblood of a low-carbon energy future
At the Paris Climate Conference in 2015, 195 countries adopted the first-ever universal, legally binding global climate deal. The agreement sets out an action plan to put the world on track to avoid dangerous climate change by limiting global warming and has been followed by a growing number of carbon net zero commitments around the world. Hydrogen is being widely touted as a key solution to phasing out our reliance on fossil fuels. But what is the so-called ‘hydrogen economy’, what benefits does it offer and what are the challenges and risks?
HYDROGEN ECONOMY
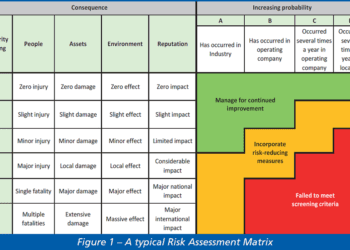
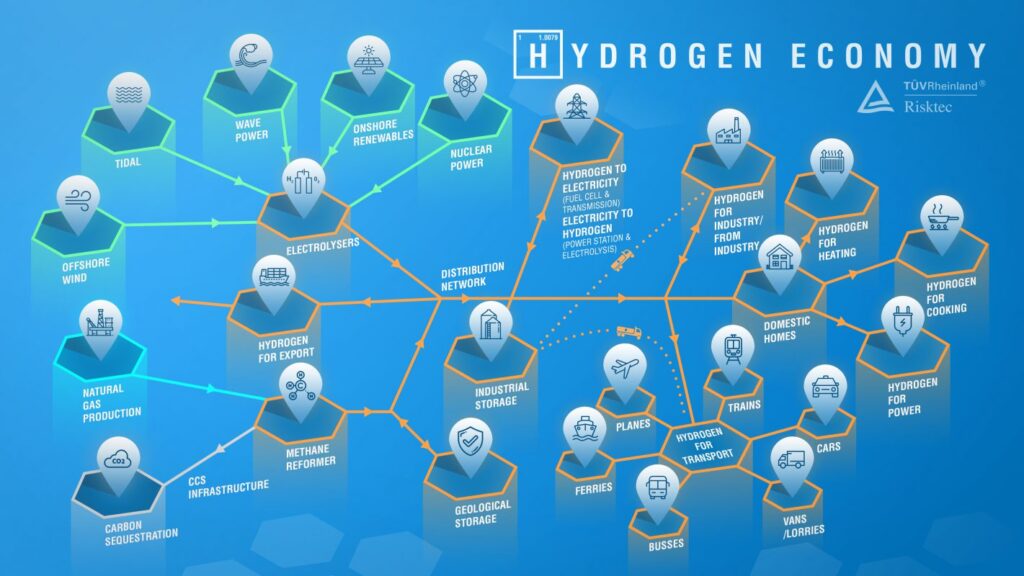
The term ‘hydrogen economy’ refers to the vision of using hydrogen as a complete, low-carbon energy source. Using hydrogen as a fuel is attractive because, whether it is burned to produce heat or combined chemically with oxygen in a fuel cell to produce electricity, the only by-product is water. Figure 1 illustrates its potential scale and diversity.
PRODUCTION
Hydrogen isn’t found in pure form on Earth, so it must be produced from other compounds such as natural gas or water. It takes energy to convert these into pure hydrogen. As such, hydrogen is really an energy carrier rather than an energy source in its own right.
‘Green hydrogen’ is generated from zero-carbon energy sources, such as renewables or nuclear. For intermittent generators, such as wind, wave and solar, converting electricity into green hydrogen provides a medium to overcome fluctuations in supply and demand.
‘Blue hydrogen’ is produced from natural gas via a process known as steam reforming. Its environmental footprint is greater than for green hydrogen as it is generated from a non-sustainable energy source that emits greenhouse gases. However, if generated on a large scale, the carbon dioxide produced can be captured and stored by Carbon Capture and Storage (CCS) facilities, buying time for zero carbon energy technologies to deliver in the longer term.
STORAGE AND DISTRIBUTION
Hydrogen can be stored as a gas, liquid or solid, the last by either reacting the hydrogen with a storage compound or through absorption into a storage material. These options imply varying volumetric efficiency and distribution solutions. For example, gaseous and liquid hydrogen can be stored underground in caverns, salt domes and depleted oil fields, which can serve as responsive large scale energy reservoirs.
Whilst hydrogen can be transported via road, rail or marine vessel, there is also the opportunity to convert existing natural gas distribution grids to supply hydrogen directly to domestic and commercial users.

USES
The most likely beneficiary of a hydrogen economy is transportation. Road transport, rail and even aviation can use hydrogen as a zero carbon fuel source, with an onboard fuel cell converting the hydrogen into electricity, for instance. Fuel cells are far more efficient than the internal combustion or jet engines they would replace. In contrast to electric vehicles, which have received much attention in recent years, hydrogen vehicles can be rapidly refuelled and have a much greater range. More generally, fuel cell technology means that hydrogen could be used to generate electricity on a national, district or consumer scale.
Finally, hydrogen has the potential to deliver a domestic heating revolution. Blended with natural gas it can supply heating and cooking appliances with only fairly simple modifications required. Better still, it could replace natural gas altogether, providing a zero-carbon domestic fuel.
HAZARDS AND RISKS
That liquid hydrogen is used as a fuel for space flight is testament to its exceptionally high energy density. In its gaseous form, hydrogen is highly flammable and easily forms an explosive mixture in air across a wide range of concentrations with a very low ignition energy.
Its small molecular size makes it highly buoyant, with a high diffusion rate and low viscosity. The first two characteristics are beneficial in terms of mitigating the risk of explosion. But its small size means that leaks are more prevalent than for natural gas. This is a key consideration in repurposing gas networks to supply hydrogen.
To compound matters, hydrogen is odourless, colourless, and tasteless and burns with an invisible flame making leak detection difficult. It can also cause embrittlement of higher carbon content metallic alloys, so care must be taken when choosing materials.
Storage presents a number of hazards. To ensure volumetric efficiency, gaseous hydrogen must be stored at very high pressures (up to 700 bar), whilst liquid hydrogen needs to be stored at very low, cryogenic temperatures.
However, broadly speaking, all of these hazards are well understood and tried and tested processes, tools and techniques exist to manage the risks effectively. For over forty years hydrogen has been used in vast quantities as an industrial chemical and fuel for space exploration.
The challenges of enabling and supporting a hydrogen economy will more likely relate to the sheer scale and proliferation of development required to make a meaningful impact on climate change. Greater still will be the challenge of overturning negative public perception. Hydrogen is perceived as a very dangerous substance, given its association with the Hindenburg airship disaster and the hydrogen bomb. Whilst the hazards of hydrogen are very real, there is no reason why hydrogen cannot be used safely. Demonstrating this to the general public through clear and effective communication will be as fundamental to the success of a hydrogen economy as the associated technical case for safety.
CONCLUSION
As a versatile, high density energy storage medium, hydrogen has the potential to play a leading role in the fight against climate change and become the lifeblood of a low carbon energy future. The success of a hydrogen economy hinges on the will and ability to scale-up the infrastructure and facilities required to achieve a meaningful impact; and reverse the public’s perception of hydrogen as a dangerous substance.
References:
1. “Hydrogen in a low-carbon economy”; Committee on Climate Change, November 2018.
2. ISO/TR 15916, Basic consideration for the safety of hydrogen systems.